Medical Device Manufacturer
VTL specializes in medical device contract manufacturing, offering comprehensive solutions from engineering services to product development and fabrication. We produce a number of medical devices and products that are either sewn, sealed, or both. Products include vacuum lock cushions used for positioning patients for imaging exams, compression garments, and environmental control products to accelerate the healing process.
Our Medical Device Solutions Include:
- Engineering services
- Product development
- Fabrication of resources
- Integration support
Types of Products Manufactured:
- Medical Inflatable Bladders
- Hospital Headpieces for Trauma Patients
- Blood Pressure Cuffs
- Compression Garments
- Containment Walls for Isolation Rooms
- Vac-lock Cushions and other Medical Positioning Devices
- Custom Medical Devices
- Custom Sewn Medical Products
- Mattress Overlays
Our expertise in medical device contract manufacturing ensures that every product meets the highest standards of quality and reliability. VTL can assist with material selection, product engineering services, quality assurance testing, tooling, RF sealing, industrial sewing, and product assembly.
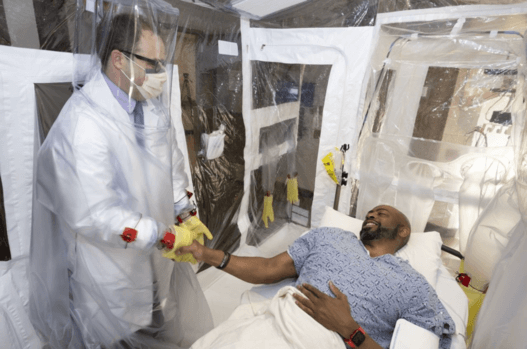
Isolation Rooms
VTL helped create temporary, low-cost, in-room, negative pressure tents that make it easier for hospitals to scale their response to infectious disease events like the COVID-19 pandemic of 2020.
- Isolation rooms like this have a clear-view PVC or TPU canopy with a high-contrast floor and RF-welded seams. Double lap hook & loop allow replacement of glove, conduit panel, and hug suit components.
- External steel frame and disposable tent with integrated glove walls and HugSuit for full patient access with significantly less need for PPE.
- Air handling is included and meets the 15 air exchanges per hour (ACH) CDC guidelines for surgical procedure and delivery rooms. The .3 micron HEPA and MERV intake filters are welded directly to the tent canopy, greatly mitigating the risk associated with user error and improved safety during the disposal process.

Vac-Lok Cushions
VTL also manufactures vacuum lock cushions used to stabilize patients in preparation for medical procedures such as MRIs and CAT Scans. Cushions form to the patient’s body contour, maintaining a patient’s position over repeated visits for improved tracking of the patient’s progress. The product is filled with styrofoam beads and then sealed shut. A valve allows for a vacuum to be pulled on the product before it is finally sealed, tested, and packed for shipment.

Why Choose VTL?
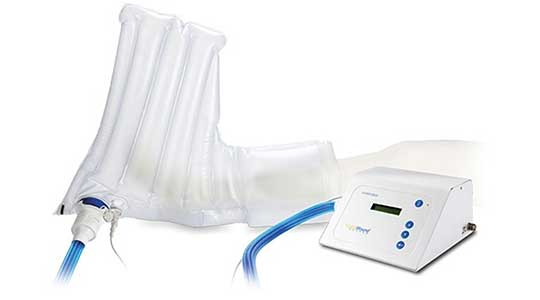
VTL produces products for the highly demanding medical industry. The inflatable boot (pictured here) enables environmentally controlled therapy of open wounds and other conditions to accelerate the healing process. Its production posed a challenge as it required a complex combination of sequenced operations and advanced tooling. VTL engineers came through and created a manufacturing method and tooling specifically for this customer. The application was a breakthrough in the treatment of slow to heal extremities and the customer and its end users are happy with the outcome. Choosing VTL for your medical device contract manufacturing needs means partnering with a company committed to excellence in every aspect of production, from material selection to final assembly.
Certifications
Vinyl Technology, LLC complies with standards required by the US government and Department of Defense that include the National Institute of Standards and Technology (NIST), The International Traffic in Arms Regulations (ITAR), Defense Federal Acquisition Regulation Supplement (DFARS) to the Federal Acquisition Regulation (FAR), STANAG 4671 and MIL-STD-6396. VTL can also fabricate products that comply with the Berry Amendment when required.
VTL’s Quality System is currently certified AS9100D and ISO9001:2015. Since 2014, VTL has implemented LEAN Manufacturing practices, Six Sigma, and Kaizen methodologies to improve operations. Vinyl Technology is an FDA registered manufacturer for medical products.
Our certifications underscore our commitment to the highest medical device contract manufacturing standards, ensuring compliance and quality for our partners.

Medical Device Contractor FAQ
What does it mean to be FDA Registered?
Licensed owners or operators of medical facilities involved in the production or distribution of medical devices are required to register annually with the FDA if the intended uses of those medical devices are for the United States. This does not signify FDA approval of products manufactured through VTL.
Where are medical devices manufactured?
VTL manufactures medical devices at our facilities in Monrovia, California.